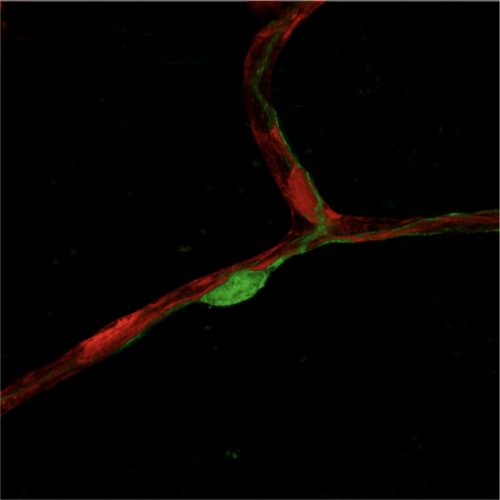
Endothelial cell–pericyte crosstalk in health and diseases
The crosstalk between endothelial cells and pericytes is essential for angiogenesis, vessel remodeling, and the maintenance of vascular homeostasis. Dysregulation of endothelial cell–pericyte interactions leads to compromised angiogenesis and vascular integrity, which are hallmark features of various diseases, including cancer, diabetic retinopathy, liver fibrosis, lung edema, and neurodegenerative disorders. Our interest lies in systematically mapping the endothelial cell–pericyte interactome in the brain, lung, and liver under both healthy and pathological conditions. By leveraging the mechanistic insights gained from this interactome, we aim to explore whether targeting specific endothelial cell–pericyte crosstalk could offer therapeutic potential for treating these diseases.
Publications: Yang et. al., Hepatology, 2021 | He et. al., Journal of Experimental Medicine, 2023 | Zhu et. al., The EMBO Journal, 2024
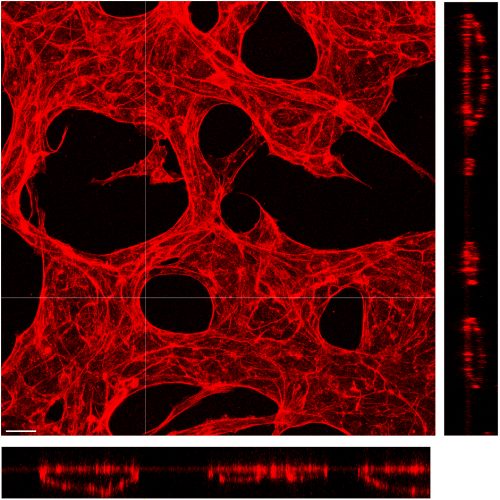
Vessel lumen size determination and maintenance
The lumen size of mature blood vessels is quite stable; however, it could undergo vessel remodeling under certain conditions, such as the enlargement of collateral vessels that connect the ischemic tissue. It is well accepted that both the blood flow and the metabolic demand of surrounding tissue play important roles in determining vessel size. However, whether there exist intrinsic pathways that actively control a vessel’s lumen size remains elusive. We are currently utilizing a microfluidic-based vessel formation system and genetic mouse models to explore the endothelial cell-intrinsic mechanisms that determine and maintain vessel diameter.
Publication: Zhang et. el., Arteriosclerosis, Thrombosis, and Vascular Biology, 2022
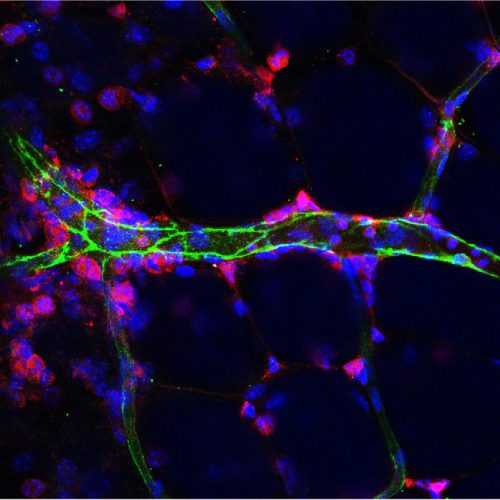
Novel mechanisms that regulate endothelial inflammatory responses
Endothelium serve as a primary interface between the blood and tissues. Upon inflammation, endothelial cells upregulate the expression of cytokines, chemokines, and adhesion molecules, which facilitate the recruitment and extravasation of immune cells into inflammatory sites. We are currently investigating novel mechanisms that regulate endothelial inflammatory response independent of the NF-κB pathway. In addition, we are collaborating with medicinal chemists in the development of novel small molecules that could specifically reduce the inflammatory responses in endothelial cells for the treatment of many inflammatory diseases.
Publication: Dai et. el., Cell Death and Disease, 2020